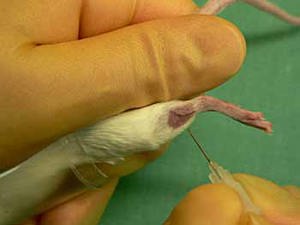
Hamster Blood Collection
вторник 05 мая admin 37
.Please read the of blood sampling page before attempting any blood sampling procedure.Also referred to as peri-orbital, posterior-orbital and orbital plexus bleeding. Retro-orbital bleeding should typically be performed as a terminal procedure. It should only be used with recovery in rare circumstances with exceptional scientific justification (e.g. Where a large blood volume is necessary or where peripheral veins are used for dosing), because of its potential impact on animal welfare.Where its use is unavoidable, retro-orbital bleeding should only be used under general or terminal.
Blood collection Cell and Biomarker Preservation Cell therapy Cell Therapy Research Solutions. APC Hamster Anti-Mouse CD3e; APC Hamster Anti-Mouse CD3e Clone 145-2C11 (RUO) Brand BD Pharmingen. Although hamster immunoglobulin isotypes have not been well defined, BD Biosciences Pharmingen has grouped Armenian and Syrian hamster IgG. Pre-rinsing blood collection equipment with an anticoagulant such as 1:1000 heparin is recommended to facilitate blood collection from the hamster due to a relatively rapid coagulation time (Edwards, 1967, Pansky et al., 1961, Timm, 1979). Lymphatic System.
Because of the severity of the adverse effects that can occur with this technique, even in skilled hands, it is essential that it is conducted only by staff members competent (practised) in the technique.Blood is collected from the venous plexus. The hamster is, the neck gently scruffed and the eye made to bulge. A capillary tube/pipette is inserted medially, laterally or dorsally. Blood is allowed to flow by capillary action into the capillary tube/pipette. The sample obtained is a mixture of venous blood and tissue fluid, and is not representative of venous blood.Blood flow can be stopped by applying gentle finger pressure to the soft tissue.
A finger should be placed over the closed eyelid for approx. 30 secondsFollowing sampling, the animals should be via an appropriate schedule one method Number of samplesIt is recommended that only one sample be taken.Sample volume0.1-0.5 mlEquipmentA glass capillary tube or Pasteur pipette.Staff resourceOne person is required to take the blood sample.Adverse effects. Tissue damage. Histological changes, abnormal clinical signs and evidence of discomfort have been reported for repeated bleeds.OtherProcedure should be carried out under terminal anaesthesia.
1 – An appropriate gavage needle will be selected for species and size of animal using “Rodent Oral Gavage Needle Guidelines” on UGA IACUC website.2 – The needle will be measured for proper length from mouth to the last rib and marked accordingly. The needle will not pass beyond this point.3 – The animal will be restrained so that the head and esophagus are in a straight line. A small amount of anesthetic gas will be used to prevent struggling and trauma.4 – The gavage needle will gently be inserted into the oral cavity behind the incisors, allowing the needle to pass as if falling by gravity. If there is any resistance, we will try again.5 – Once the gavage needle is placed correctly, we will slowly inject to a maximum volume (0.1ml/10g of body weight).6 – The gavage needle will be removed using a straight, steady motion.7 – The animal will be monitored for signs of labored breathing, sudden lethargy or poor mucous membrane color.
If signs are present, the animal will be euthanized immediately. 1 – A 6-8 French feeding tube as appropriate for the size of the rabbit will be selected.2 – The tube will be measured for proper length from the tip of the nose to the last rib and marked accordingly. The tube will not beyond this point.3 – The rabbit will be restrained, ideally in a box restrainer.4 – The tube will be lubricated with sterile lubricant and inserted into the nasal cavity until the tube begins to pass. If there is any resistance, we will try again.5 – We will check for proper tube placement by putting the outer tip of the tube in bowl of water and confirming that there are no air bubbles. We will also inject a small amount of water (2ml) and aspirate to verify greenish-brown stomach contents.6 – The solution will be injected slowly to a maximum volume (10ml/kg of body weight).7 – The end of the tube will be pinched before removing it to prevent stomach contents from draining into the oral-pharyngeal region.8 – The animal will be monitored for signs of distress such as gasping or frothing at the mouth. If signs are present, the animal will be euthanized immediately. 1 – The mouse will be restrained appropriately.
The saphenous vein on the outside of the lower back leg will be located.2 – The fur over the vein will be clipped and sterile lubricant gel will be applied.3 – The vein will be occluded by applying pressure just above the knee.4 – The vessel will be punctured perpendicularly using a 25g needle.5 – We will allow the puncture to form a drop and then collect using a microhematocrit tube or pipettor. If necessary, we will flex the foot to increase the flow of blood.6 – We will collect no more than the maximum volume of blood (200ul for a 25g mouse every 2 weeks).7 – Pressure will be applied to the puncture site until bleeding dissipates. 1 – The rat will be restrained appropriately. The saphenous vein on the outside of the lower back leg will be located.2 – The fur over the vein will be clipped and sterile lubricant gel will be applied.3 – The vein will be occluded by applying pressure just above the knee.4 – The vessel will be punctured perpendicularly using a 20g-24g needle.5 – We will allow the puncture to form a drop and then collect using a microhematocrit tube or pipettor. If necessary, we will flex the foot to increase the flow of blood.6 – We will collect no more than the maximum volume of blood (1.6ml for a 250g rat every 2 weeks).7 – Pressure will be applied to the puncture site until bleeding dissipates.
1 – The hamster will be restrained appropriately. The saphenous vein on the outside of the lower back leg will be located.2 – The fur over the vein will be clipped and sterile lubricant gel will be applied.3 – The vein will be occluded by applying pressure just above the knee.4 – The vessel will be punctured perpendicularly using a 20g-24g needle.5 – We will allow the puncture to form a drop and then collect using a microhematocrit tube or pipettor. If necessary, we will flex the foot to increase the flow of blood.6 – We will collect no more than the maximum volume of blood (750ul for a 125g hamster every 2 weeks).7 – Pressure will be applied to the puncture site until bleeding dissipates. 1 – The gerbil will be restrained appropriately. The saphenous vein on the outside of the lower back leg will be located.2 – The fur over the vein will be clipped and sterile lubricant gel will be applied.3 – The vein will be occluded by applying pressure just above the knee.4 – The vessel will be punctured perpendicularly using a 22g-25g needle.5 – We will allow the puncture to form a drop and then collect using a microhematocrit tube or pipettor. If necessary, we will flex the foot to increase the flow of blood.6 – We will collect no more than the maximum volume of blood (500ul for an 80g gerbil every 2 weeks).
1 – The guinea pig will be restrained appropriately.The saphenous vein on the outside of the lower back leg will be located.2 – The fur over the vein will be clipped and sterile lubricant gel will be applied.3 – The vein will be occluded by applying pressure just above the knee.4 – The vessel will be punctured perpendicularly using a 20g-24g needle.5 – We will allow the puncture to form a drop and then collect using a microhematocrit tube or pipettor. If necessary, we will flex the foot to increase the flow of blood.6 – We will collect no more than the maximum volume of blood (5ml for a 1000g guinea pig every 2 weeks).7 – Pressure will be applied to the puncture site until bleeding dissipates.
1 – The mouse will be restrained appropriately. The pedal vein on the top of the hind foot will be located.2 – The vein will be occluded by applying light pressure on the ankle. Sterile lubricant gel but do not apply alcohol will be applied.3 – The vessel will be punctured by using a 25g needle, being careful to not go through the foot.4 – We will allow the puncture to form a drop and then collected using a microhematocrit tube or pipettor. Volumes are usually very small.5 – Pressure will be applied to the puncture site until bleeding dissipates.
1 – The rat will be restrained appropriately. The pedal vein on the top of the hind foot will be located.2 – The vein will be occluded by applying light pressure on the ankle. Sterile lubricant gel but do not apply alcohol will be applied.3 – The vessel will be punctured by using a 25g needle, being careful to not go through the foot.4 – We will allow the puncture to form a drop and then collect using a microhematocrit tube or pipettor.
Volumes are usually very small.5 – Pressure will be applied to the puncture site until bleeding dissipates. 1 – The appropriate size lancet will be selected (3mm for small amount of blood, 4mm for mice 6 months).2 – The mouse will be restrained in a firm scruff with your non-dominant hand, ensuring that the skin over the face is held taunt. 1 – The tail vessels will be dilated by placing the tail in warm water (37-40 C) or placing the entire animal under a heat lamp (at least 25-30cm from the bulb) for 2-5 minutes. We will carefully monitor the mouse for signs of distress during heat exposure.2 – The mouse will be restrained so that its tail is accessible.3 – The veins that run bilaterally along the sides of the tail will be located.4 – We will make a quick perpendicular puncture of the vein using a 25-28g needle.
NOTE: Using a needle is safer than using a blade which could inadvertently dissect the tail.5 – Drops of blood will be collected using a microhematocrit tube or drop directly into a collection tube. Do not “milk” the tail.6 – Pressure will be applied to the puncture site until bleeding dissipates. 1 – The tail vessels will be dilated by placing the tail in warm water (37-40 C) or placing the entire animal under a heat lamp (at least 25-30cm from the bulb) for 2-5 minutes.
We will carefully monitor the rat for signs of distress during heat exposure.2 – The rat will be restrained so that the underside of its tail is accessible.3 – The veins that run bilaterally along the sides of the tail will be located.4 – We will make a quick perpendicular puncture of the vein using a 22-25g needle. NOTE: Using a needle is safer than using a blade which could inadvertently dissect the tail.5 – Drops of blood will be collected using a microhematocrit tube or drop directly into a collection tube.
Do not “milk” the tail.6 – Pressure will be applied to the puncture site until bleeding dissipates. 1 – The tail artery will be dilated by placing the tail in warm water (37-40 C) or placing the entire animal under a heat lamp (at least 25-30cm from the bulb) for 2-5 minutes. We will carefully monitor the rat for signs of distress during heat exposure.2 – The rat will be restrained so that the underside of its tail is accessible.3 – The artery that runs on the ventral (bottom) side of the tail will be located.4 –The tail will be bent so that the needle can be inserted parallel to the tail, just under the skin. The first puncture will be about half-way down the tail so that it can move cranially if the first collection attempt is unsuccessful.5 – A 25-28g needle will be inserted, bevel up, into the artery, approximately 20 degrees from the skin. Visualize the penetration and insert cranially about 2 mm.6 – We will aspirate using the syringe plunger or alternately use a needle without a syringe and collect using a microhematocrit tube.7 – The needle will be removed and pressure will be applied to the puncture site until bleeding dissipates.
1 – The rodent will be anesthetized if it is over 3 weeks of age.2 – The animal will be restrained and the tail will be pulled gently to its full length for an accurate measurement.3 – Sharp, sterile scissors or scalpel will be used. 0.5-1.0mm of the fleshy tip of the tail will be removed.
A maximum of 5mm can be taken through repeated bleedings.4 – Small drops of blood will be collected by using a microhematocrit tube or dropping directly into a collection tube. Do not “milk” the tail.5 – Pressure will be applied to the tail tip until bleeding dissipates.6 – We will monitor the animal until it has fully recovered from anesthesia.7 – For short-term repeat sampling, the clot will often be removed and the tail does not have to be re-snipped.8 – When using a blade and a hot bead sterilizer, we will allow the blade to cool slightly between cuts and use for no more than 5 cuts total. 1 – The rat will be anesthetized or restrain securely.2 – The rat will be placed on its back on solid surface (or scruff and hold on its back if not anesthetized.)3 – With one hand, we will extend the rat’s head gently backward and turned slightly toward the rat’s left shoulder. With the other hand, we will push the right forelimb down and put slight pressure on the rat’s right shoulder region to expose the neck and occlude the jugular vein.4 – This region will be shaved and cleaned with alcohol or surgical scrub.5 – We will palpate for the “v” at the sternoclavicular junction. The jugular vein lies in this region.
It may or may not be visible.6 – We will use a 23g x 1” needle attached to a 1ml or 3ml syringe. 1 – The rabbit will be restrained securely. Vasodilation can be induced by administering acepromazine 5-10 minutes before blood collection.2 – The fur will be clipped from the outer ears, if this is necessary to visualize the vessels. The veins (lateral vessels) can be used for smaller volumes of blood, however larger volumes are best collected from the artery (central vessel).3 – The ear will be cleaned with alcohol or surgical scrub.4 – We will use a 21g or higher (smaller) needle (either a needle and syringe, or a butterfly needle with tubing).5 – With the non-dominant hand, we will gently pull the ear towards us, to keep the ear taut. If a vein is used, the vein will be occluded at the base of the ear. If the artery is used, we will make sure not to occlude the artery.6 – With your dominant hand, the needle will be inserted, at an angle of 20 degrees or less, into the vessel. A flash of blood indicates the needle is in the vessel.
Once the needle is in the vessel, we will carefully decrease the angle so that the needle is almost parallel to the vessel and aspirated slowly.7 – When the blood sample has been collected, we will slowly remove the needle at the same angle as entry.8 – Pressure will be applied over the artery for at least 3 minutes to provide hemostasis. 1 – The mouse will be scruffed and we will tent the skin overlying the back of the neck.2 – We will inject the animal in the base of the triangle formed by this tent by using the smallest possible needle that will easily pass the material, one no larger than 25g.3 – We will retract the syringe plunger to verify that no blood is aspirated before making the injection.4 – No more than 250ul will be injected at a single site for an average 25g mouse (10ul per gram). If a larger volume is required, we will use multiple sites along the back but inject no more than 1 ml total (40ul per gram). 1 – The rat will be scruffed and we will tent the skin overlying the back of the neck.2 – We will inject the animal in the base of the triangle formed by this tent by using the smallest possible needle that will easily pass the material, one no larger than 25g.3 – We will retract the syringe plunger to verify that no blood is aspirated before making the injection.4 – No more than 1.25ml will be injected at a single site for an average 250g rat (5ul per gram). If a larger volume is required, we will use two sites along the back but inject no more than 2.5mls total (10ul per gram). 1 – The gerbil will be scruffed and we will tent the skin overlying the back of the neck.2 – We will inject the animal in the base of the triangle formed by this tent by using the smallest possible needle that will easily pass the material, one no larger than 25g.3 – We will retract the syringe plunger to verify that no blood is aspirated before making the injection.4 – No more than 750ul will be injected at a single site for an average 75g gerbil (10ul per gram).
If a larger volume is required, we will use multiple sites along the back but inject no more than 3mls total (40ul per gram). 1 – The hamster will be scruffed and we will tent the skin overlying the back of the neck.2 – Inject the animal in the base of the triangle formed by this tent. Use the smallest possible needle that will easily pass the material, one no larger than 25g.3 – We will retract the syringe plunger to verify that no blood is aspirated before making the injection.4 – No more than 1.25mls will be injected at a single site for an average 125g hamster (10ul per gram). If a larger volume is required, we will use multiple sites along the back but inject no more than 5mls total (40ul per gram). 1 – The guinea pig will be scruffed and we will tent the skin overlying the back of the neck.2 – Inject the animal in the base of the triangle formed by this tent. Use the smallest possible needle that will easily pass the material, one no larger than 23g.3 – We will retract the syringe plunger to verify that no blood is aspirated before making the injection.4 – No more than 5mls will be injected at a single site for an average 1000g guinea pig (5ml per kg). If a larger volume is required, we will use two sites along the back but inject no more than 10mls total (10mls per kg).
1 – The rabbit will be restrained appropriately and tent a section of dorsal skin without using the area directly over the neck since this is the site where the animal is scruffed for restrained.2 – The animal will be injected in the base of the triangle formed by this tent by using the smallest possible needle that will easily pass the material, one no larger than 21g.3 – We will retract the syringe plunger to verify that no blood is aspirated before making the injection.4 – No more than 4mls will be injected at a single site for an average 4kg rabbit (1ml per kg). If a larger volume is required, we will use two sites along the back but inject no more than 8mls total (2mls per kg). 1 – Using the non-dominant hand, the mouse will be scruffed, securing the entire loose skin along the back in the palm of the hand. The tail will be secured by using the smallest finger. We will turn our palm upward, so that the mouse is lying on its back with its abdomen exposed.2 – We will tip the animals head downward to allow the internal organs to move forward toward the chest cavity.3 – The needle will be inserted at a 15-20 degree angle into the animal’s lower right quadrant by using the smallest needle that will easily pass the material, one no larger than 25g. Once the needle has penetrated the abdominal cavity, it will be kept straight to prevent slicing the abdominal organs with the sharp needle tip.4 – We will retract the syringe plunger to verify that no blood, feces or urine is present before making the injection. If contaminants are drawn up into the syringe, we will try again with a new needle and syringe making sure not to inject contaminants into the abdominal cavity as this will likely result in a serious infection.5 – No more than 500ul will be injected for an average 25g mouse (20ul per gram).
If a larger volume is required, up to 2mls total (80ul per gram) can be injected but is not recommended. 1 – Using your non-dominant hand, the rat will be scruffed or restrain in a device being sure to secure the tail. With the rat lying on its back, the abdomen will be exposed.2 – We will tip the animals head downward to allow the internal organs to move forward toward the chest cavity.3 – The needle will be inserted at a 15-20 degree angle into the animal’s lower right quadrant by using the smallest needle that will easily pass the material, one no larger than 23g.
Once the needle has penetrated the abdominal cavity, it will be kept straight to prevent slicing the abdominal organs with the sharp needle tip.4 – We will retract the syringe plunger to verify that no blood, feces or urine is present before making the injection. If contaminants are drawn up into the syringe, we will try again with a new needle and syringe making sure not to inject contaminants into the abdominal cavity as this will likely result in a serious infection.5 – No more than 2.5mls will be injected for an average 250g rat (10ul per gram). If a larger volume is required, up to 5mls total (20ul per gram) can be injected but is not recommended. 1 – Using the non-dominant hand, the gerbil will be scruffed, securing the entire loose skin along the back in the palm of the hand. The tail will be secured by using the smallest finger. We will turn our palm upward, so that the gerbil is lying on its back with its abdomen exposed.2 – We will tip the animals head downward to allow the internal organs to move forward toward the chest cavity.3 – The needle will be inserted at a 15-20 degree angle into the animal’s lower right quadrant by using the smallest needle that will easily pass the material, one no larger than 25g. Once the needle has penetrated the abdominal cavity, it will be kept straight to prevent slicing the abdominal organs with the sharp needle tip.4 – We will retract the syringe plunger to verify that no blood, feces or urine is present before making the injection.
If contaminants are drawn up into the syringe, we will try again with a new needle and syringe making sure not inject to contaminants into the abdominal cavity as this will likely result in a serious infection.5 – No more than 1.5mls will be injected for an average 75g gerbil (20ul per gram). If a larger volume is required, up to 6mls total (80ul per gram) can be injected but is not recommended. 1 – Using the non-dominant hand, the hamster will be scruffed securing the entire loose skin along the back in the palm of the hand. We will turn our palm upward, so that the hamster is lying on its back with its abdomen exposed.2 – We will tip the animals head downward to allow the internal organs to move forward toward the chest cavity.3 – The needle will be inserted at a 15-20 degree angle into the animal’s lower right quadrant by using the smallest needle that will easily pass the material, one no larger than 23g. Once the needle has penetrated the abdominal cavity, it will be kept straight to prevent slicing the abdominal organs with the sharp needle tip.4 – We will retract the syringe plunger to verify that no blood, feces or urine is present before making the injection.
If contaminants are drawn up into the syringe, we will try again with a new needle and syringe making sure not inject to contaminants into the abdominal cavity as this will likely result in a serious infection.5 – No more than 2.5mls will be injected for an average 120g hamster (20ul per gram). If a larger volume is required, up to 10mls total (80ul per gram) can be injected but is not recommended.
1 – Using the non-dominant hand, the guinea pig will be scruffed or restrained in a device. With the guinea pig lying on its back, the abdomen will be exposed.2 – We will tip the animals head downward to allow the internal organs to move forward toward the chest cavity.3 – The needle will be inserted at a 15-20 degree angle into the animal’s lower right quadrant by using the smallest needle that will easily pass the material, one no larger than 23g. Once the needle has penetrated the abdominal cavity, it will be kept straight to prevent slicing the abdominal organs with the sharp needle tip.4 – We will retract the syringe plunger to verify that no blood, feces or urine is present before making the injection.
If contaminants are drawn up into the syringe, we will try again with a new needle and syringe making sure not inject to contaminants into the abdominal cavity as this will likely result in a serious infection.5 – No more than 10mls will be injected for an average 1000g guinea pig (10ml per kg). If a larger volume is required, up to 20mls total (20ml per kg) can be injected but is not recommended. 1 – The tail vessels will be dilated by placing it in warm water (37-40 C) or placing the entire animal under a heat lamp (at least 25-30cm from the bulb) for 2-5 minutes. The mouse will be carefully monitored for signs of distress during heat exposure.2 – The mouse will be restrained so that its tail is accessible. A commercial restrainer is often very useful for this procedure.3 – The veins will be located that run bilaterally along the sides of the tail by slightly twist the tail to access the vein. Injections will start half way down the tail so that we can move cranially if the first injection attempt is unsuccessful.4 – A needle will be inserted with the bevel upward at an approximately 20 degree angle.
(Alternately, it might be easier to bend the tail to adjust your angle of entry.) The smallest needle, no larger than 26g, will be used.5 – Once the needle has penetrated the vein, we will decrease the angle and direct cranially about 2mm.6 – We will retract the syringe plunger to verify that blood is aspirated before making the injection. For an average 25g mouse, no more than 125ul will be injected for a bolus injection (5ul per gram) or 625ul for a slow drip (25ul per gram). The vein should blanch with injection and there should be no noticeable swelling at the injection site.7 –The needle will slowly be removed and pressure will be applied to the injection site until bleeding dissipates.
1 – The tail vessels will be dilated by placing it in warm water (37-40 C) or placing the entire animal under a heat lamp (at least 25-30cm from the bulb) for 2-5 minutes. The rat will be carefully monitored for signs of distress during heat exposure.2 – The rat will be restrained so that its tail is accessible. A commercial restrainer is often very useful for this procedure.3 – The veins will be located that run bilaterally along the sides of the tail by slightly twist the tail to access the vein. These vessels can be difficult to visualize. It is often easier to visualize these veins near the tail base.4 – A needle will be inserted with the bevel upward at an approximately 20 degree angle.
The smallest needle, no larger than 21g, will be used.5 – Once the needle has penetrated the vein, we will decrease your angle and direct cranially about 2mm.6 – We will retract the syringe plunger to verify that blood is aspirated before making the injection. For an average 250g rat, no more than 1.25ml will be injected for a bolus injection (5ul per gram) or 5ml for a slow drip (20ul per gram).
The vein should blanch with injection and there should be no noticeable swelling at the injection site.7 – the needle will slowly be removed and apply pressure to the injection site until bleeding dissipates. 1 – The fur on the top of the ear will be clipped to aide in visualization. Alcohol will be used to clean the site.2 –The vessels will be occluded by gently holding off at the base of the ear.3 – A needle, no larger than 23 grams, will be inserted with the bevel upward at an approximately 20 degree angle.4 – The syringe plunger will be retracted to verify that no blood is aspirated before making the injection. No more than 8mls for a bolus injection (2ml per kg) or 40mls for a slow drip (10mls per kg). The vein should blanch with injection and there will be no noticeable swelling at the injection site.5 – The needle will be slowly removed and pressure applied to the injection site until bleeding dissipates.6 – For repeat injections or large volumes, a catheter will be inserted. Rat Intramuscular Injections1 – The rat will be restrained. We will flex the back hind limb so that the quadriceps muscle is clearly visible.2 – A needle, no larger than 25 grams, will be inserted perpendicular to the skin, directly into the muscle.3 – The syringe plunger will be retracted to verify that no blood is aspirated before making the injection.
No more than 25ul per site (0.1ul per gram) or 50ul total (0.2ul per gram) of material will be injected for an average 250g rat.4 –The needle will be slowly removed and pressure applied to the injection site until bleeding dissipates. 1 – The guinea pig will be restrained.
We will flex the back hind limb so that the quadriceps muscle is clearly visible.2 – A needle, one no larger than 25g, will be inserted perpendicular to the skin, directly into the muscle.3 – The syringe plunger will be retracted to verify that no blood is aspirated before making the injection. No more than 100ul of material will be injected.4 – The needle will be slowly removed and pressure will be applied to the injection site until bleeding dissipates. 1 – The rabbit will be restrained. We will either flex the back leg toward the body and inject in the front part of the back thigh or inject in the lumbar muscles that run beside the spine, just above the pelvis. The back of the rear thigh muscle will not be injected.2 – A needle, one no larger than 25g and ½ to 1 inches long, will be selected and inserted perpendicular to the skin, directly into the muscle to a depth of 7-10mm.3 – Retract the syringe plunger to verify that no blood is aspirated before making the injection.
For an average 4kg rabbit, do not inject more than 1ml per site (0.25ml per kg) or 2mls total (0.5ml per kg).4 – Slowly remove the needle and apply pressure to the injection site until bleeding dissipates. 1 – The guinea pig will be anesthetized or restrained. The dorsal thoracic or lumbar region will be selected for the injection.2 – The area will be shaved and cleaned with alcohol.3 – A needle, one no larger than 25g, will be selected and injected with no more than 100ul per site (50ul recommended).4 – A needle will be inserted just under the skin, at a very shallow angle, (approximately 15-20 degrees) that will administer material to create a small bleb in the skin. If a bleb does not form or quickly dissipates, the injection will be SQ not ID.5 – There should be minimal bleeding with this technique.6 – Up to 10 injection sites will be used.
Download Pokemon Stadium 2 Nintendo 64(N64) ROM ✅ and play Pokemon Stadium 2 on Phone, PC or MAC! Pokemon Stadium 2 ROM download is available to play for Nintendo 64. This Pokemon game is the US English version at EmulatorGames.net exclusively. Download Pokemon Stadium 2 ROM and use it with an emulator. Play online N64 game on desktop PC, mobile, and tablets in maximum quality. DOWNLOAD Pokemon Stadium 2 ROM (Download Manager) DOWNLOAD Pokemon Stadium 2 ROM (Direct) PLAY Pokemon Stadium 2 ONLINE. In order to be able to play this game you need an emulator installed. See the full list of available Nintendo 64 emulators for this game.  Download Pokemon Stadium 2 ROM for Nintendo 64(N64) and Play Pokemon Stadium 2 Video Game on your PC, Mac, Android or iOS device!
Download Pokemon Stadium 2 ROM for Nintendo 64(N64) and Play Pokemon Stadium 2 Video Game on your PC, Mac, Android or iOS device!
1 – The rabbit will be anesthetized or restrained. A dorsal caudal thoracic or lumbar region will be selected for injection.2 – The area will be shaved and cleaned with alcohol.3 – A needle, one no larger than 25g, will be selected and injected with no more than 100ul per site (50ul recommended).4 – A needle will be inserted just under the skin, at a very shallow angle, (approximately 15-20 degrees) that will administer material to create a small bleb in the skin. If a bleb does not form or quickly dissipates, the injection will be SQ not ID.5 – There should be minimal bleeding with this technique.6 – Up to 10 injection sites will be used. 1 – The rat will be restrained. We will flex the back hind limb so that the quadriceps muscle is clearly visible.2 – A needle, no larger than 25 grams, will be inserted perpendicular to the skin, directly into the muscle.3 – The syringe plunger will be retracted to verify that no blood is aspirated before making the injection. No more than 25ul per site (0.1ul per gram) or 50ul total (0.2ul per gram) of material will be injected for an average 250g rat.4 –The needle will be slowly removed and pressure applied to the injection site until bleeding dissipates. 1 – The guinea pig will be anesthetized or restrained.
The dorsal thoracic or lumbar region will be selected for the injection.2 – The area will be shaved and cleaned with alcohol.3 – A needle, one no larger than 25g, will be selected and injected with no more than 100ul per site (50ul recommended).4 – A needle will be inserted just under the skin, at a very shallow angle, (approximately 15-20 degrees) that will administer material to create a small bleb in the skin. If a bleb does not form or quickly dissipates, the injection will be SQ not ID.5 – There should be minimal bleeding with this technique.6 – Up to 10 injection sites will be used.